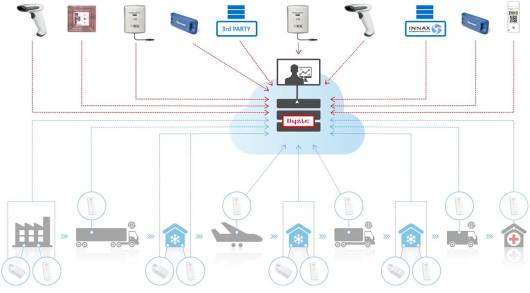
The IoT (internet of things) is now commonly talked about in almost all industry sectors. Everyone is talking about connecting devices and using the data to do something meaningful with it – whether it’s in healthcare, logistics, manufacturing, retail, or any other system that could benefit from a ‘feedback loop’.
According to the Gartner hype cycle, IoT is almost at the peak of inflated expectation (see chart below), but in my opinion, it is already over its peak – IoT solutions have existed for over a decade, with Dyzle’s cold chain monitoring solution being evidence of this, which has been established for a while.
So now that the hype is over, what is next? What can we realistically expect from IoT now?

Well one thing is for sure – we already have the beginnings of a new hype cycle with new network technologies like LoRa and Sigfox. They promise low cost hardware and long battery life, as outlined in my earlier article ‘IoT in the last mile for drug delivery: balancing technology and cost’. As I mentioned in this, new IoT technology solutions are being launched all the time, but networks are not always fully rolled out, so coverage in practice is often limited. It could be some years before such networks become ubiquitous and provide the same level of coverage as mobile networks do currently, and especially indoors.
So LoRa and Sigfox are possibly at the stage where RFID (radio frequency identification) technology was in the 1990s. There were high expectations and many possible use cases, but in reality, it didn’t really become viable for widespread use until about 20 years later. Is this the same case now?
Returning to the question of what’s next with IoT, we have to consider the types of applications – whether it’s simple or complex IoT.
IoT simple use cases
There are many examples of simple use cases for IoT where there are growing multiple market applications already rolled out. These include:
- Garbage bins in the city, where they can indicate whether they are full or empty, enabling city resources for waste collection to be optimized, and potentially reducing costs.
- Smart vending machines, which offer benefits to both customers (such as reserving drinks before going), as well as retailers and operators, who can replenish supplies only when the vending machine indicates it is close to being empty (see this example).
- Smart parking, which can tell you whether is a parking space, whether in a car park or off-street, is free before even getting there (such as this one in Perth, Australia, or this one in Milton Keynes, UK).
Many of these simple IoT applications are characterized by the fact that low reliability is not an issue, that there are low amounts of data, and few measurements per day. The challenge though is network coverage and also indoor signal coverage.
IoT complex use cases
Complex IoT use cases include:
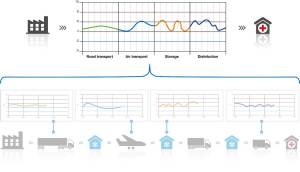
- Monitoring of critical parameters of goods in a supply chain (such as temperature sensitive pharmaceutical and food products).
- Predictive maintenance in a plant (often as part of an industrial automation system), enabling spare parts being delivered to machines before any failure or downtime occurs.
- Patient monitoring (such as the scenarios I described in a previous article on IoT for ‘hospital-at-home’)
The characteristics of such complex IoT systems are that they need robust solutions, reliability of data (they can’t miss a data sample because the measurements are often of critical parameters), and in the case of ‘excursions’, they need to be triggered to provide even more data and possibly even different types of additional data.
Achieving this is not yet possible with current network technologies. The question then is going to be: will NB-IoT (narrowband IoT), when it becomes a global standard, meet the expectations of such complex IoT use cases? That is yet to be seen.
So what should you expect from IoT today?
Looking at the Gartner hype cycle, it suggests that IoT will take between 2-5 years to come to its plateau of productivity. I agree with this. When NB-IoT networks and the technology (ie: the chipsets), become more mature and widely available, then this will really see IoT take off in a big way, especially for some of the complex IoT use cases. This is because they’ll then be able to deliver high value compared to the low cost of implementation.
But there is no real need to wait for that to happen. There are already multiple IoT solutions available on existing technologies (some might say old) such as GSM, GPRS and Wi-Fi; in most cases their robustness is proven. And let’s not forget, that even the space shuttle was using Intel 8086 chips for almost 20 years – so it wasn’t exactly using leading edge technology, but instead using older, tried and tested chips.
The existing solutions also help meet market conformance standards – such as computer system validation (CSV), a requirement in the pharmaceutical industry.
There is a lot more mileage left in these solutions for entire supply chains both in the food and pharmaceutical industries, where the value can be harvested probably for at least the next five years.
When these systems have served their purpose, and created their value, the next stage will be more advanced IoT solutions creating even more value. Especially since you’ve already built up a wealth of experience because you have the experience from implementing systems using existing technologies and learning how to benefit from it.
So, yes, the hype is over. And now is the time to reap the benefits of IoT using existing technologies over the next five years or so. That way, you’ll be much more ready for the hype cycle of the next generation technologies to reach their peak of inflated expectations.